Multiple Choice
Which of the following equations represents the Nernst equation?
A) 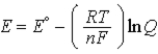
B) 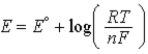
C) 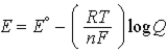
D) 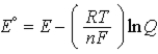
E) 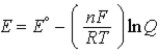
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q76: Write a balanced chemical equation for the
Q77: When the following oxidation-reduction reaction in acidic
Q78: Which of the following is true for
Q79: Balance the following half-reaction occurring in an
Q80: Balance the following oxidation-reduction reaction occurring in
Q81: What half-reaction occurs at the cathode during
Q82: Use the following standard reduction potentials to
Q83: Which of the following is the balanced
Q84: Given the following two half-reactions,write the overall
Q86: Calculate the cell potential at 25 °C