Multiple Choice
What is the standard entropy change for the following reaction? 4 N2(g)
+ 12 H2(g)
→
8 NH3(g) 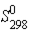 (J/mol⋅K)
(J/mol⋅K)
191) 5
130) 6
192) 3
A) -794.8 J/K⋅mol-rxn
B) 794.8 J/K⋅mol-rxn
C) 2948.8 J/K⋅mol-rxn
D) -2948.8 J/K⋅mol-rxn
E) -129.8 J/K⋅mol-rxn
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q31: _ changes only occur in the direction
Q32: The standard free energy change for a
Q33: When a real gas is compressed from
Q34: For any process,the change in entropy of
Q35: What is the S0 for ozone if
Q37: A 100 mL sample of water is
Q38: For a chemical reaction,if Δ<sub>r</sub>G° = 0,then
Q39: If Δ<sub>r</sub>G° > 0 for a reaction
Q40: The change in entropy for any process
Q41: Which of the following changes lead to