Multiple Choice
Calculate ΔrG° at 25.0 °C for the reaction below. 2 Na(s) + 2 H2O( 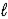 ) → 2 NaOH(aq) + H2(g)
) → 2 NaOH(aq) + H2(g)
Given: ΔrH° = −366.6 kJ/mol-rxn and ΔrS° = −154.2 J/K⋅mol-rxn.
A) -371.2 kJ/mol-rxn
B) -320.6 kJ/mol-rxn
C) -215.4 kJ/mol-rxn
D) +371.2 kJ/mol-rxn
E) +4634.9 kJ/mol-rxn
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: Thermodynamics can be used to determine all
Q7: Calculate the enthalpy of vaporization of water
Q8: Which of the following is the first
Q9: What is the sign of ΔH (system)and
Q10: Using the given data,determine ΔrG° at 298
Q12: Which of the following statements about entropy
Q13: A flask containing helium gas is released
Q14: Which of the following is true of
Q15: In any chemical process,energy must be conserved.This
Q16: Which of the following is the second