Multiple Choice
The standard free energy change associated with the dissolution of ammonium nitrate in water is -6.73 kJ/mol at 298.15 K. NH4NO3(s) 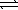 NH4NO3(aq)
NH4NO3(aq)
Which of the following is the equilibrium constant for the reaction? (R = 8.314 J/K⋅mol)
A) 1.9 × 10-3
B) 6.6 × 10-2
C) 1.0
D) 15
E) 5.2 × 102
Correct Answer:

Verified
Correct Answer:
Verified
Q15: In any chemical process,energy must be conserved.This
Q16: Which of the following is the second
Q17: Which of the following is correct for
Q18: For which of the following reactions will
Q19: The dissolution of ammonium nitrate occurs spontaneously
Q21: Does the formation of complex molecules such
Q22: Calculate the standard entropy change for the
Q23: A change of state occurring in a
Q24: Calculate Δ<sub>r</sub>G° for the reaction below at
Q25: If a cube of ice at 0