Multiple Choice
The Ksp of silver bromide is 5.4 × 10−13 at 298 K. AgBr(s) 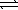 Ag+(aq) + Br−(aq)
Ag+(aq) + Br−(aq)
What is ΔrG°? (R = 8.314 J/K⋅mol)
A) −3.0 × 101 kJ/mol
B) −5.87 kJ/mol
C) 5.87 kJ/mol
D) 3.0 × 101 kJ/mol
E) 7.0 × 101 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q25: If a cube of ice at 0
Q26: In which of the following reactions is
Q27: Hydrogen gas is prepared by electrolysis of
Q28: Given the following data: S(g)+ O<sub>2</sub>(g)→ SO<sub>2</sub>(g)<br>Δ<sub>r</sub>G°
Q29: The standard entropy for the formation of
Q31: _ changes only occur in the direction
Q32: The standard free energy change for a
Q33: When a real gas is compressed from
Q34: For any process,the change in entropy of
Q35: What is the S0 for ozone if