Multiple Choice
Calculate ΔrG° for the reaction below at 425 °C, 2 HI(g) 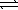 H2(g) + I2(g) ; K = 0.018.(R = 8.314 J/K⋅mol)
H2(g) + I2(g) ; K = 0.018.(R = 8.314 J/K⋅mol)
A) 6.12 × 103 kJ/mol-rxn
B) 1.05 × 104 kJ/mol-rxn
C) 1.42 × 104 kJ/mol-rxn
D) 2.33 × 104 kJ/mol-rxn
E) 3.34 × 105 kJ/mol-rxn
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q50: The total entropy of the universe is
Q51: For a reaction,Δ<sub>r</sub>H° = -208.8 kJ and
Q52: Which of the following relationships is not
Q53: For the reaction given below,ΔH<sup>0</sup> = −1516
Q54: What is the equilibrium constant for reaction
Q56: ΔG° = 0 for a reaction indicates
Q57: For the reaction Br<sub>2</sub>(l)→ 2Br(g),_.<br>A) ΔH is
Q58: If a chemical reaction is exothermic but
Q59: Which of the following is the third
Q60: For a certain reversible process,q = 88.06