Multiple Choice
Potassium hydrogen phthalate (KHP) is used to standardize sodium hydroxide.If 35.39 mL of NaOH(aq) is required to titrate 0.8246 g KHP to the equivalence point,what is the concentration of the NaOH(aq) ? (The molar mass of KHP = 204.2 g/mol) HC8H4O4-(aq) + OH-(aq) 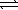 C8H4O42-(aq) + H2O(
C8H4O42-(aq) + H2O(  )
)
A) 0.02318 M
B) 0.05705 M
C) 0.0859 M
D) 0.1141 M
E) 0.1429 M
Correct Answer:

Verified
Correct Answer:
Verified
Q29: A certain weak base B has a
Q30: Calculate the pH of a solution made
Q31: What is the pH of a buffer
Q32: You have 75.0 mL of 0.17 M
Q33: A volume of 25.0 mL of 0.100
Q35: A 1.0 liter solution contains 0.25 M
Q36: A 50.00-mL solution of 0.0350 M ethylamine
Q37: What mass of solid NaCH<sub>3</sub>CO<sub>2</sub> (molar mass
Q38: Hyperventilation can cause your blood pH to
Q39: What is the pH of an aqueous