Multiple Choice
The concentration of calcium carbonate in a saturated aqueous solution at 25°C is 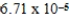 M.What is the Ksp of this sparingly soluble salt?
M.What is the Ksp of this sparingly soluble salt?
A) 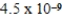
B) 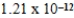
C) 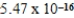
D) 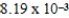
E) 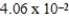
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: How many moles of solid NaF would
Q21: Why do salts containing basic anions have
Q22: Which of the following combinations would be
Q23: What is the solubility product expression for
Q24: An aqueous solution contains 0.010 M bromide
Q26: A 50.00-mL solution of 0.0400 M hydrofluoric
Q27: What is the pH of a buffer
Q28: A 5.0 × 10<sup>-4</sup> M solution of
Q29: A certain weak base B has a
Q30: Calculate the pH of a solution made