Multiple Choice
The hydroxide ion concentration of a saturated solution of Fe(OH) 2 is 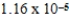 M.What is the solubility product constant for Fe(OH) 2?
M.What is the solubility product constant for Fe(OH) 2?
A) 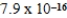
B) 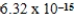
C) 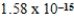
D) 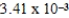
E) 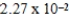
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q65: A 50.00-mL solution of 0.0426 M trimethylamine
Q66: What is the pH at the equivalence
Q67: Two important biological buffer systems control pH
Q68: What is the maximum hydroxide-ion concentration that
Q69: What will be the pH of the
Q71: What is the pH of a buffer
Q72: How many moles of solid NaF would
Q73: If 25 mL of 0.750 M HCl
Q74: If 20 mL of 0.10 M NaOH
Q75: What is the minimum mass of Na<sub>2</sub>CO<sub>3</sub>