Multiple Choice
Calculate the molar concentration of uncomplexed Zn2+(aq) in a solution that contains 0.17 M Zn(NH3) 42+ and 0.3775 M NH3 at equilibrium.Kf for Zn(NH3) 42+ is 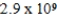 .
.
A) 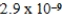 M
M
B) 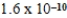 M
M
C) 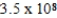 M
M
D) 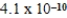 M
M
E) 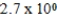 M
M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q76: When a weak base is titrated with
Q77: All of the following statements concerning acid-base
Q78: If 0.50 L of a buffer containing
Q79: If 500 mL of 1.4 × 10<sup>-6</sup>
Q81: The solubility of manganese(II)carbonate is 5.4 ×
Q82: What is the molar solubility of silver(I)iodide
Q83: What is the concentration of silver(I)ion in
Q85: What is the concentration of Cd<sup>2+</sup>(aq)in a
Q86: A buffer contains 0.50 mol NH<sub>4</sub><sup>+</sup> and
Q134: Which of the following indicators is most