Multiple Choice
Given the following reactions, AgBr(s) 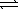 Ag+(aq) + Br-(aq)
Ag+(aq) + Br-(aq)
Ksp = 5.4 × 10-13
Ag+(aq) + 2 CN-(aq) 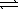 Ag(CN) 2-(aq)
Ag(CN) 2-(aq)
Kf = 1.2 × 1021
Determine the equilibrium constant for the reaction below.
AgBr(s) + 2 CN-(aq) 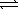 Ag(CN) 2-(aq) + Br-(aq)
Ag(CN) 2-(aq) + Br-(aq)
A) 4.5 × 10-34
B) 1.5 × 10-9
C) 6.5 × 108
D) 1.2 × 1021
E) 2.2 × 1033
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q56: The pH of a solution at the
Q57: When mixed in appropriate amounts,each of the
Q58: If the ratio of acid to base
Q59: What is the pH of a buffer
Q61: What is the pH of the buffer
Q62: What molar ratio of acetic acid to
Q63: What mass of sodium hydroxide must be
Q64: Suppose 50.00 mL of 2.0 × 10<sup>-5</sup>
Q65: A 50.00-mL solution of 0.0426 M trimethylamine
Q105: In which of the following solutions would