Multiple Choice
Given the two equilibria below, Ag(NH3) 2+(aq) 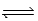 Ag+(aq) + 2NH3(aq) ; Kd = 5.9 × 10-8
Ag+(aq) + 2NH3(aq) ; Kd = 5.9 × 10-8
AgCN(s) 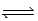 Ag+(aq) + CN−(aq) ; Ksp =
Ag+(aq) + CN−(aq) ; Ksp = 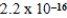 what is K for the following equilibrium?
what is K for the following equilibrium?
AgCN(s) + 2NH3(aq)  Ag(NH3) 2+(aq) + CN-(aq)
Ag(NH3) 2+(aq) + CN-(aq)
A) 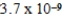
B) 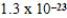
C) 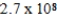
D) 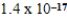
E) 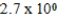
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: How many moles of HCl must be
Q2: Which of the following mathematical expressions is
Q4: Which of the following is the common
Q5: What is the pH of a solution
Q6: If the ratio of acid to base
Q7: What is the molar solubility of Mn(OH)<sub>2</sub>(s)in
Q8: Consider the titration of 300.0 mL of
Q9: What is the value of the dissociation
Q10: A 50.0 mL sample of 0.155 M
Q11: The K<sub>sp</sub> of BaSO<sub>4</sub> is 1.1 ×