Multiple Choice
Given the following equilibrium constants, Zn4IO3) 2 Ksp = 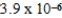 Zn(NH3) 42+ Kf =
Zn(NH3) 42+ Kf = 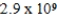 determine K for the dissolution of the sparingly soluble salt Zn(IO3) 2 in aqueous ammonia (shown below) .
determine K for the dissolution of the sparingly soluble salt Zn(IO3) 2 in aqueous ammonia (shown below) .
Zn(IO3) 2(s) + 4NH3(aq) ⇌ Zn(NH3) 42+(aq) + 2IO3-(aq)
A) 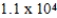
B) 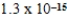
C) 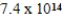
D) 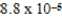
E) 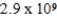
Correct Answer:

Verified
Correct Answer:
Verified
Q12: What is the molar solubility of solid
Q13: The K<sub>sp</sub> of Ca(OH)<sub>2</sub> is 5.5 ×
Q14: What color change is exhibited by phenolphthalein
Q15: The solubility of strontium carbonate (SrCO<sub>3</sub>)in water
Q16: If 25 mL of 0.10 M NaOH
Q18: Which acid-base combination is depicted by this
Q19: What is the minimum concentration of Cd<sup>2+</sup>
Q20: How many moles of solid NaF would
Q21: Why do salts containing basic anions have
Q22: Which of the following combinations would be