Multiple Choice
At 50°C the autoionization constant for pure water,Kw,is 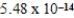 .What is the H3O+ concentration in pure water at 50°C?
.What is the H3O+ concentration in pure water at 50°C?
A) 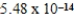 M
M
B) 1.01 × 10-7 M
C) 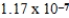 M
M
D) 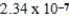 M
M
E) 1.01 × 10-14 M
Correct Answer:

Verified
Correct Answer:
Verified
Q58: Brønsted-Lowry acid-base reactions are restricted to only
Q59: The pH of a solution at 25
Q60: Which of the following substances is never
Q61: Which of the following ionic compounds does
Q62: Aqueous solutions of ammonia (NH<sub>3</sub>)and hydrogen cyanide
Q64: What is the H<sub>3</sub>O<sup>+</sup> concentration in 0.0072
Q65: Which of the following expressions is not
Q66: Carbonic acid is a diprotic acid,H<sub>2</sub>CO<sub>3</sub>,with K<sub>a1</sub>
Q67: The equilibrium constant,K<sub>a</sub>,for a monoprotic acid (benzoic
Q68: In the reaction CaO(s)+ CO<sub>2</sub>(g)→ CaCO<sub>3</sub>(s),<br>A) O<sup>2-</sup>