Multiple Choice
The complete reaction of an acid and base is as follows. HNO3(aq) + LiOH(aq) → H2O( 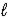 ) + LiNO3(aq)
) + LiNO3(aq)
What is the equilibrium constant for the net ionic reaction at 25 °C?
A) 1.01 × 10-14
B) 1.01 × 10-7
C) 1.01 × 107
D) 1.01 × 1014
E) more information required
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q71: Calculate the pH of a 1.7 M
Q72: Which of the following is the correct
Q73: At 25 °C,all of the following ions
Q74: Which one of the following aqueous solutions
Q75: Which of the following is true of
Q77: Given the following equilibrium constants, K<sub>a</sub> (HSO<sub>4</sub><sup>-</sup>)=
Q78: All of the following compounds are acids
Q79: What is the pH of a 0.33
Q80: When a Lewis acid and a Lewis
Q81: Hydrofluoric acid has a pK<sub>a</sub> value of