Multiple Choice
What is the equilibrium pH of an initially 4.9 M solution of hypoiodous acid,HOI,at 25°C (Ka = 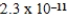 ; Kw = 1.01 × 10-14) ?
; Kw = 1.01 × 10-14) ?
A) 4.97
B) 7.00
C) 5.12
D) 4.82
E) 8.34
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q81: Hydrofluoric acid has a pK<sub>a</sub> value of
Q82: Arrange the following acids in order of
Q83: All of the following species behave as
Q84: Identify from the following list of molecules
Q85: What is K<sub>a</sub> at 25°C for the
Q87: What is the conjugate base of [Fe(H<sub>2</sub>O)<sub>6</sub>]<sup>3+</sup>(aq)?<br>A)
Q88: What is the pH of the solution
Q89: Which of the following is the correct
Q90: What is the pH of a 0.42
Q91: According to the Brønsted-Lowry definition,a base<br>A) increases