Short Answer
The chemical equations below show the reaction of Al(OH)3 as a Lewis acid and as a Lewis base,respectively.
Al(OH)3(s)+ OH-(aq) 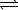 Al(OH)4-(aq)
Al(OH)4-(aq)
Al(OH)3(s)+ 3 H3O+(aq) 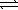 Al3+(aq)+ 3 H2O(
Al3+(aq)+ 3 H2O( 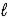 )
)
Substances that can behave as either Lewis acids or bases are called ________ substances.
Correct Answer:

Verified
Correct Answer:
Verified
Q43: Which of the following is the correct
Q44: What is the equilibrium concentration of H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>
Q45: A solution has a hydroxide ion concentration
Q46: What is the hydronium-ion concentration in a
Q47: What is the hydroxide-ion concentration of a
Q49: What is the hydronium-ion concentration of a
Q50: Which are the Brønsted-Lowry bases in the
Q51: Consider the K<sub>a</sub> values for the following
Q52: Which of the following species cannot act
Q53: When a Lewis acid combines with a