Multiple Choice
For the reaction A → B + C,which of the following equations corresponds to the integrated expression for a second-order decomposition reaction?
A) 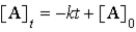
B) 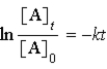
C) 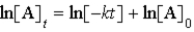
D) 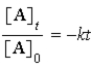
E) 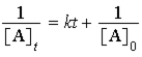
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q24: The rate constant of a first-order decomposition
Q25: Consider the following proposed mechanism.If this mechanism
Q26: A catalyst _.<br>A) is used up in
Q27: The decomposition of formic acid follows first-order
Q28: Molecules must overcome a barrier called the
Q30: A second-order reaction starts with an initial
Q31: Draw the reaction coordinate diagram for an
Q32: For the reaction provided,the rate of disappearance
Q33: For the overall reaction A + 2B
Q34: In a reaction coordinate diagram,reacting molecules are