Multiple Choice
Hydrogen peroxide decomposes into water and oxygen in a first-order process. H2O2(aq) → H2O( 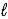 ) + 1/2 O2(g)
) + 1/2 O2(g)
At 20.0 °C,the half-life for the reaction is 3.92 × 104 seconds.If the initial concentration of hydrogen peroxide is 0.52 M,what is the concentration after 7.00 days?
A) 1.2 × 10-5 M
B) 0.034 M
C) 0.074 M
D) 0.22 M
E) 0.52 M
Correct Answer:

Verified
Correct Answer:
Verified
Q1: For the reaction A + 2B →
Q2: Which of the following units are consistent
Q3: Which of the following expressions does not
Q4: In general,as temperature increases,the rate of a
Q5: For a certain reaction of the general
Q7: For a certain reaction of the general
Q8: Termolecular elementary steps are rare.Why?
Q10: If a reaction is second-order with respect
Q11: In a first-order reaction,the half-life is 133
Q44: A first-order chemical reaction is observed to