Multiple Choice
For the hypothetical reaction aA → products,the experimental data showed the following behavior (below) .What is the reaction order with respect to reactant A? 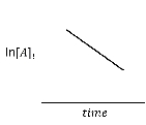
A) zero order
B) first order
C) second order
D) third order
E) fourth order
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q51: Below is a proposed mechanism for the
Q51: How many mechanistic steps are depicted by
Q52: The rate constant at 366 K for
Q53: For the second-order reaction below,the initial concentration
Q54: The rate constant for a first-order reaction
Q57: For the formation of 1 mol of
Q58: What is the name given to a
Q59: Circle the catalyst in the following mechanism.
Q60: A first-order reaction is 40.0% complete at
Q61: For a chemical reaction,the activation energy for