Multiple Choice
The Arrhenius equation, 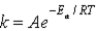 expresses the dependence of the rate constant on the reaction temperature.The slope of a plot of ln(k) versus 1/T is equal to
expresses the dependence of the rate constant on the reaction temperature.The slope of a plot of ln(k) versus 1/T is equal to
A) 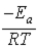
B) 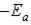
C) 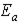
D) 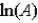
E) 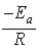
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q32: For the reaction provided,the rate of disappearance
Q33: For the overall reaction A + 2B
Q34: In a reaction coordinate diagram,reacting molecules are
Q35: The rate constant for a reaction at
Q36: Which of the following statements is correct
Q38: The decomposition of phosphine,PH<sub>3</sub>,follows first-order kinetics. 4
Q39: Nitrogen dioxide reacts with carbon monoxide to
Q40: The _ of an elementary step is
Q41: Which of the given relationships correctly compares
Q42: For the overall reaction 2A + B