Multiple Choice
Which of the following equations illustrates the formation of an aqueous solution of KF from its elements in their standard states?
A) K+(g) + F−(g) → K+(aq) + F−(aq)
B) KF(s) → KF(aq,1 m)
C) K+(g) + F−(g) → KF(s)
D) K(s) + 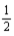 F2(g) → KF(s)
F2(g) → KF(s)
E) K(s) + 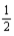 F2(g) → KF(aq,1 m)
F2(g) → KF(aq,1 m)
Correct Answer:

Verified
Correct Answer:
Verified
Q69: What partial pressure of oxygen gas is
Q70: What is the vapor pressure at 20°C
Q71: A concentrated nitric acid solution has a
Q72: Which of the following statements about soaps
Q73: The solubility of 1-pentanol in water is
Q74: What is the boiling-point change for a
Q75: The vapor pressure of water at 90°C
Q76: If the concentration of sodium carbonate in
Q78: Henry's law states that the solubility of
Q79: Colloids represent a state intermediate between a