Multiple Choice
Henry's Law constant is 0.0013 mol/kg⋅bar and 0.034 mol/kg⋅bar for O2 and CO2 respectively at 25°C.What pressure of CO2 is required to achieve the same solubility as 0.711 bar of O2?
A) 0.0 bar
B) 19.0 bar
C) 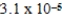 bar
bar
D) 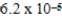 bar
bar
E) 37.0 bar
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q26: What is the molality of ethanol (C<sub>2</sub>H<sub>5</sub>OH)in
Q27: Assuming ideal behavior,which of the following aqueous
Q28: What is the concentration unit used in
Q29: The Henry's law constant for N<sub>2</sub> in
Q30: Colloids are described by all of the
Q32: What mass of ethylene glycol,when mixed with
Q33: All of the following are colloidal dispersions
Q34: What volume of a 0.758 M solution
Q35: A solution consisting of 0.278 mol of
Q36: The lattice enthalpy of LiCl is −834