Multiple Choice
What is the length of the diagonal (in atomic radii) along the face of a face-centered cubic unit cell?
A) 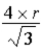
B) 2 × r
C) 4 × r
D) 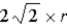
E) r
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q48: Which of the following elements might be
Q49: A low-melting solid readily dissolves in water
Q50: Nickel has a face-centered cubic cell,and its
Q51: Lithium chloride crystallizes in a face-centered cubic
Q52: Polonium (atomic mass 209.0 g/mol)crystallizes in a
Q54: For a metal that crystallizes in a
Q55: Cesium bromide crystallizes in a primitive cubic
Q56: Gold (atomic mass 197 g/mol),with an atomic
Q57: Elements that have their highest energy electrons
Q58: Strontium oxide has a face centered cubic