Multiple Choice
What is the simplest formula of the compound represented by the unit cell provided below? 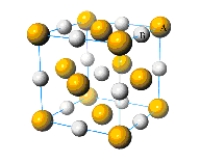
A) AB
B) AB2
C) AB3
D) A2B3
E) A2B4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: If a metal crystallizes in a body-centered
Q34: What is the distance,in atomic radii,along any
Q35: Iron(II)sulfide has a primitive cubic unit cell
Q36: Which two of the following materials are
Q37: The metal vanadium crystallizes in a body-centered
Q39: According to the below phase diagram,what process
Q40: Copper crystallizes in a face-centered cubic lattice.The
Q41: Above a substance's _ temperature,it is not
Q42: On the phase diagram below,which point corresponds
Q43: Calcium sulfide has a face-centered cubic unit