Multiple Choice
Which intermolecular force or bond is responsible for the density of H2O(s) being less than that of H2O( 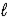 ) ?
) ?
A) London dispersion forces
B) hydrogen bonding
C) ionic bonding
D) covalent bonding
E) dipole/induced dipole forces
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q43: Normal boiling point is defined as<br>A) the
Q44: Arrange HF,HCl,and HBr in increasing order of
Q45: Ammonia (NH<sub>3</sub>)is used as a refrigerant.At its
Q46: At 80.0 °C,water has an equilibrium vapor
Q47: Which of the following molecules has the
Q49: Which of the following intermolecular forces or
Q50: Hydrogen bonding is present in all of
Q51: Which of the following nonpolar molecules has
Q52: Capillary action is _<br>A) resistance to flow.<br>B)
Q53: Which of the following molecules has the