Multiple Choice
List all the intermolecular forces present in pure acetone. 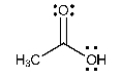
A) hydrogen bonding only
B) dipole-dipole force only
C) dipole-dipole force and London dispersion forces
D) hydrogen bonding and London dispersion forces
E) hydrogen bonding,dipole-dipole force,and London dispersion forces
Correct Answer:

Verified
Correct Answer:
Verified
Q59: Which one of the following molecules will
Q60: Make a sketch to show the hydrogen
Q61: The heat of vaporization of benzene (C<sub>6</sub>H<sub>6</sub>)is
Q62: Which of the following liquids would you
Q63: When a glass tube with a small
Q64: The best explanation for the existence of
Q65: Which of the following bonds can potentially
Q66: London dispersion forces are the only significant
Q68: When a water molecule forms a hydrogen
Q69: Which of the following molecules has the