Multiple Choice
Which of the following is not a valid resonance structure for N3-?
A) 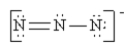
B) 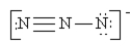
C) 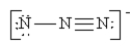
D) 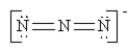
E) all are correct
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q79: What is the molecular geometry around the
Q80: The given representation of an atom is
Q81: Atoms having equal or nearly equal electronegativities
Q82: Which of the following are the correct
Q83: Place the following molecules in order from
Q84: The Lewis structure of which of the
Q85: One resonance structure for OCN<sup>-</sup> ion is
Q86: Which of the following pairs of compounds
Q87: How many different molecules have the molecular
Q89: Three nonequivalent Lewis structures for carbonyl sulfide,SCO,are