Multiple Choice
Three possible structures of C2H2Cl2 are shown below.Which of these molecules are polar? 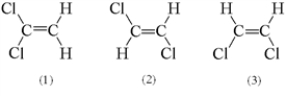
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 2 and 3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q46: What are the approximate F−Br−F bond angles
Q47: What is the molecular geometry around carbon
Q48: What is the total number of electrons
Q49: Use VSEPR theory to predict the electron-pair
Q50: Which of the following Lewis structures for
Q52: The molecular geometry of a molecule whose
Q53: What is the electron-pair geometry around an
Q54: What is the formal charge on the
Q55: The molecule H<sub>2</sub>S has<br>A) 2 bonding pairs
Q56: Which of the following exhibits resonance?<br>A) SCl<sub>4</sub><br>B)