Multiple Choice
Which of the following sets of quantum numbers is allowed?
A) n = 2, 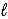 = 1,
= 1, 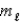 = +1/2,ms = -1/2
= +1/2,ms = -1/2
B) n = 3, 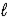 = 2,
= 2, 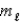 = +1,ms = +1
= +1,ms = +1
C) n = 4, 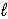 = 1,
= 1, 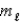 = 0,ms = -1/2
= 0,ms = -1/2
D) n = 4, 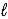 = 3,
= 3, 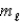 = -1,ms = 0
= -1,ms = 0
E) n = 5, 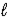 = 2,
= 2, 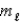 = +2,ms = -1
= +2,ms = -1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q50: Which of the following statements is true?<br>A)
Q51: The element _ has the electron configuration
Q52: What 2+ ion has the following ground
Q53: According to the Aufbau principle,which of the
Q54: Electron _ is defined as the energy
Q56: The f-block elements are also referred to
Q57: Hund's rule states that the most stable
Q58: The small,but important,energy differences between 3s,3p,and 3d
Q59: Which of the following species has the
Q60: Place the following atoms in order of