Multiple Choice
Which of the following sets of quantum numbers is not allowed?
A) n = 4, 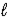 = 2,and
= 2,and 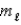 = -2
= -2
B) n = 6, 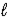 = 1,and
= 1,and 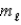 = 1
= 1
C) n = 5, 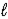 = 4,and
= 4,and 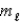 = -4
= -4
D) n = 2, 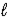 = 1,and
= 1,and  = -3
= -3
E) n = 6, 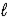 = 3,and
= 3,and 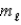 = -2
= -2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q26: What is the energy of a photon
Q27: Which of the following regions of the
Q28: What is the de Broglie wavelength of
Q30: The Bohr model predicts that the energy
Q32: How many values are there for the
Q33: Which type of experiment demonstrates that an
Q34: Which of the following orbital can be
Q35: For which of the following electron transitions
Q36: The contribution for which de Broglie is
Q36: A device operates at a frequency of