Multiple Choice
Which of the following properties is associated with the value of the 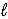 quantum number?
quantum number?
A) the shape of an orbital
B) the size of an orbital
C) the number of electrons in an orbital
D) the energy of an orbital
E) the orientation in space of an orbital
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: Which type of experiment demonstrates that an
Q34: Which of the following orbital can be
Q35: For which of the following electron transitions
Q36: A device operates at a frequency of
Q37: How many f orbitals are in the
Q39: Which of the following sets of quantum
Q40: What is the energy per mole of
Q41: According to Heisenberg's _ principle,it is impossible
Q42: What is the total number of orbitals
Q43: If a cordless phone operates at a