Multiple Choice
The thermochemical equation for the combustion of butane is givenn below. C4H10(g) + 13/2 O2(g) → 4 CO2(g) + 5 H2O( 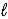 )
)
ΔrH° = -2877 kJ/mol-rxn
What is the enthalpy change for the reaction below?
8 CO2(g) + 10 H2O( 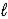 ) → 2 C4H10(g) + 13 O2(g)
) → 2 C4H10(g) + 13 O2(g)
A) +1439 kJ/mol-rxn
B) +2877 kJ/mol-rxn
C) -5754 kJ/mol-rxn
D) -2877 kJ/mol-rxn
E) +5754 kJ/mol-rxn
Correct Answer:

Verified
Correct Answer:
Verified
Q28: The heat of vaporization of benzene,C<sub>6</sub>H<sub>6</sub>,is 30.7
Q29: Given the thermochemical equation 4AlCl<sub>3</sub>(s)+ 3O<sub>2</sub>(g)→ 2Al<sub>2</sub>O<sub>3</sub>(s)+
Q30: _ is used to measure the energy
Q31: Which of the following processes will result
Q32: Calculate ΔU of a gas for a
Q34: When 10.0 g of KOH is dissolved
Q35: When 66.0 g of an unknown metal
Q36: Why are you at greater risk from
Q37: When 0.265 mol of a weak base
Q38: A bomb calorimeter has a heat capacity