Multiple Choice
Determine the heat of evaporation of carbon disulfide, CS2( 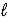 ) → CS2(g)
) → CS2(g)
Given the enthalpies of reaction below.
C(s) + 2 S(s) → CS2( 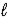 )
)
ΔrH° = +89.4 kJ/mol-rxn
C(s) + 2 S(s) → CS2(g)
ΔrH° = +116.7 kJ/mol-rxn
A) -206.1 kJ
B) -27.3 kJ
C) +27.3 kJ
D) +206.1 kJ
E) +1.31 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: The thermochemical equation for the combustion of
Q8: Determine the standard enthalpy of formation of
Q9: Heat capacity is defined as<br>A) the amount
Q10: Which of these physical changes would require
Q11: 44.0 g of ice at -20.0 °C
Q13: If 35.0 g H<sub>2</sub>O at 22.7 °C
Q15: In a reaction,the values of ∆H and
Q16: How much energy is gained by copper
Q17: If 46.1 g of copper at 11.6
Q103: Which of the following is an endothermic