Multiple Choice
Determine the standard enthalpy of formation of Fe2O3(s) given the thermochemical equations below. Fe(s) + 3 H2O( 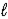 ) → Fe(OH) 3(s) + 3/2 H2(g)
) → Fe(OH) 3(s) + 3/2 H2(g)
ΔrH° = +160.9 kJ/mol-rxn
H2(g) + 1/2 O2(g) → H2O( 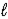 )
)
ΔrH° = -285.8 kJ/mol-rxn
Fe2O3(s) + 3 H2O( 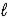 ) → 2 Fe(OH) 3(s)
) → 2 Fe(OH) 3(s)
ΔrH° = +288.6 kJ/mol-rxn
A) -252.6 kJ/mol-rxn
B) +163.7 kJ/mol-rxn
C) -824.2 kJ/mol-rxn
D) +33.2 kJ/mol-rxn
E) + 890.6 kJ/mol-rxn
Correct Answer:

Verified
Correct Answer:
Verified
Q20: Which of the following thermodynamic quantities are
Q22: What is the minimum mass of ice
Q23: Calculate the energy in the form of
Q24: Dry ice converts directly from a solid
Q26: Acetylene,C<sub>2</sub>H<sub>2</sub>,is a gas used in welding.The molar
Q27: Determine Δ<sub>r</sub>H° for the following reaction,2 NH<sub>3</sub>(g)+
Q28: The heat of vaporization of benzene,C<sub>6</sub>H<sub>6</sub>,is 30.7
Q29: Given the thermochemical equation 4AlCl<sub>3</sub>(s)+ 3O<sub>2</sub>(g)→ 2Al<sub>2</sub>O<sub>3</sub>(s)+
Q30: _ is used to measure the energy
Q32: It is relatively easy to change the