Multiple Choice
Which of the following reactions corresponds to the thermochemical equation for the standard molar enthalpy of formation of solid zinc nitrate?
A) Zn2+(aq) + 2NO3-(aq) → Zn(NO3) 2(s)
B) Zn(OH) 2(s) + 2HNO3(aq) → Zn(NO3) 2(s) + 2H2O( 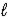 )
)
C) Zn(s) + N2(g) + 3O2(g) → Zn(NO3) 2(s)
D) Zn(s) + 2HNO3(aq) → Zn(NO3) 2(s) + H2(g)
E) Zn(s) + 2N(g) + 6O(g) → Zn(NO3) 2(s)
Correct Answer:

Verified
Correct Answer:
Verified
Q36: Why are you at greater risk from
Q37: When 0.265 mol of a weak base
Q38: A bomb calorimeter has a heat capacity
Q39: Using the following thermochemical data: 2Sm(s)+ 6HF(g)→
Q40: Internal energy and enthalpy are state functions.What
Q42: The specific heat capacity of water(liquid)is 4.18
Q43: Which one of the following statements is
Q44: Many homes are heated using natural gas.The
Q45: What is the change in internal energy
Q46: CaO(s)reacts with water to form Ca(OH)<sub>2</sub>(aq).If 6.50