Multiple Choice
If 5.00 g Br2 and 1.10 g NH3 react according to the equation below,what is the maximum mass of ammonium bromide produced? 3 Br2( 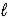 ) + 8 NH3(g) → 6 NH4Br(s) + N2(g)
) + 8 NH3(g) → 6 NH4Br(s) + N2(g)
A) 3.06 g
B) 6.13 g
C) 12.9 g
D) 4.74 g
E) 8.43 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: The percent yield of a chemical reaction
Q19: What is the pH of 4.1 ×
Q20: A 2.197 g sample of a compound
Q21: How many grams of dioxygen are required
Q22: Potassium hydrogen carbonate decomposes according to the
Q24: Chlorine was passed over 1.06 g of
Q25: What mass of iron can be produced
Q26: A dilute solution is prepared by transferring
Q27: Soft drink bottles are made of polyethylene
Q28: Nitric oxide is made from the oxidation