Multiple Choice
Aspirin is produced by the reaction of salicylic acid (M = 138.1 g/mol) and acetic anhydride (M = 102.1 g/mol) . C7H6O3(s) + C4H6O3( 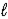 ) → C9H8O4(s) + C2H4O2(
) → C9H8O4(s) + C2H4O2( 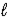 )
)
If 2.04 g of C9H8O4 (M = 180.2 g/mol) is produced from the reaction of 3.03 g C7H6O3 and 4.01 g C4H6O3,what is the percent yield?
A) 28.8%
B) 29.0%
C) 50.9%
D) 51.6%
E) 67.3%
Correct Answer:

Verified
Correct Answer:
Verified
Q6: One step in the isolation of pure
Q7: How many moles of sodium bromide can
Q8: Which of the following is true about
Q9: In combustion analysis,the gases produced by the
Q10: How many moles of Mg<sub>3</sub>P<sub>2</sub>(s)can be produced
Q12: The pH of an aqueous sodium hydroxide
Q13: What is the molarity of an NaI
Q14: Sulfur dioxide will react with water to
Q15: Under certain conditions the reaction of ammonia
Q16: Calculate the number of moles of O<sub>2</sub>