Multiple Choice
Which one of the statements below is false concerning the following reaction: NH3(g) + H2O 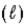
 NH4+(aq) + OH−(aq)
NH4+(aq) + OH−(aq)
A) The double arrows indicate that ammonia,NH3,is only very slightly soluble in water.
B) The reaction is reversible.
C) When NH3 is added to H2O,NH4+ and OH− ions are produced in a 1:1 ratio.
D) When solutions of NH4+ and OH- are mixed,some ammonia is produced.
E) Ammonia partially reacts with water.
Correct Answer:

Verified
Correct Answer:
Verified
Q47: Identify a true statement about oxidation numbers.<br>A)
Q48: A solution is a homogeneous mixture composed
Q49: _ acid is produced in a larger
Q50: Which of the following ions is most
Q51: What is the net ionic equation for
Q53: All of the following compounds are soluble
Q54: The oxidation number of chlorine is highest
Q55: Metals react with oxygen gas to produce
Q56: In the reaction of acetic acid with
Q57: Which of the following equations is not