Multiple Choice
Write a balanced equation for the reaction that occurs when hydrogen sulfate ion behaves as a Brønsted-Lowry acid in water.
A) HSO4-(aq) + H2O( 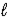 )
) 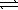 H2SO4(aq) + H3O+(aq)
H2SO4(aq) + H3O+(aq)
B) SO42-(aq) + H2O( 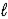 )
) 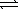 HSO4-(aq) + OH-(aq)
HSO4-(aq) + OH-(aq)
C) HSO4-(aq) + H2O( 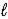 )
) 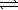 SO42-(aq) + H3O+(aq)
SO42-(aq) + H3O+(aq)
D) HSO4-(aq) + H2O( 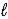 )
) 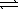 H2SO4(aq) + OH-(aq)
H2SO4(aq) + OH-(aq)
E) H2SO4(aq) + H2O(  )
)  HSO4-(aq) + H3O+(aq)
HSO4-(aq) + H3O+(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q29: Which of the following is a strong
Q30: Give the name of an acidic oxide
Q31: What are the smallest whole number coefficients
Q32: All of the following compounds are insoluble
Q33: What is the oxidation number of P
Q35: When aqueous solutions of calcium iodide,CaI<sub>2</sub>,and silver
Q36: What are the spectator ions in the
Q37: When solutions of barium iodide and lithium
Q38: The products of the complete combustion of
Q39: Which of the following compounds acts as