Multiple Choice
What is the balanced equation for carbonate ion (CO32-) acting as a Brønsted base in a reaction with water?
A) CO32-(aq) + 3 H2O( 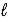 )
) 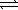 CO44-(aq) + 2 H3O+(aq)
CO44-(aq) + 2 H3O+(aq)
B) CO32-(aq) + H2O( 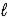 )
) 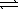 HCO3-(aq) + OH-(aq)
HCO3-(aq) + OH-(aq)
C) CO32-(aq) + H2O( 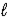 )
) 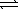 CO2(g) + 2 OH-(aq)
CO2(g) + 2 OH-(aq)
D) CO32-(aq) + 2 H2O( 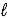 )
) 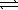 HCO3-(aq) + H3O+(aq)
HCO3-(aq) + H3O+(aq)
E) CO32-(aq) + H2O( 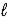 )
) 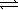 H2CO42-(aq)
H2CO42-(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q2: A precipitate is expected when an aqueous
Q3: Which of the following equations best represents
Q4: A(n)_ agent gains electrons in an oxidation-reduction
Q5: If an aqueous solution of _ is
Q6: Which of the following reactions best describes
Q8: What is the oxidation number of each
Q9: What is the smallest whole number coefficient
Q10: Which of the following is a weak
Q11: A precipitate will form when aqueous Pb(NO<sub>3</sub>)<sub>2</sub>
Q12: Nitric acid is the product of the