Multiple Choice
Which of the following is the correct net ionic equation for the reaction between aqueous ammonia and hydrobromic acid?
A) HBr(aq) + NH3(aq) → NH4Br(aq)
B) H+(aq) + OH-(aq) → H2O( 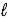 )
)
C) HBr(aq) + OH-(aq) → Br-(aq) + H2O( 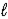 )
)
D) H+(aq) + NH3(aq) → NH4+(aq)
E) H+(aq) + Br-(aq) + NH3(aq) → NH4+(aq) + Br-(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q58: Which of the following compounds is insoluble
Q59: Write a balanced chemical equation for the
Q60: Which of the following chemical equations is
Q61: Which anion will form a precipitate with
Q62: A precipitate will form when aqueous nickel(II)chloride
Q64: Which of the following products will form
Q65: What base results when K<sub>2</sub>O reacts with
Q66: Which species in the reaction below undergoes
Q67: Which species is reduced in the reaction
Q68: Metal oxides react with water to produce