Multiple Choice
Write a balanced net ionic equation for the reaction of aqueous solutions of baking soda (NaHCO3) and acetic acid.
A) HCO3-(aq) + CH3CO2H(aq) → CH3CO2-(aq) + H2O( 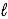 ) + CO2(g)
) + CO2(g)
B) 2 NaHCO3(aq) + CH3CO2H(aq) → 2 Na2CO3(aq) + CH4(aq) + 2H2O( 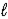 ) + CO2(g)
) + CO2(g)
C) NaHCO3(aq) + H+(aq) → H2CO3(s) + Na+(aq)
D) HCO3-(aq) + H+(aq) → H2O( 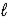 ) + CO2(g)
) + CO2(g)
E) HCO3-(aq) + H+(aq) → H2CO3(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q38: The products of the complete combustion of
Q39: Which of the following compounds acts as
Q40: Ionic and molecular compounds that form ions
Q41: Which anion will form a precipitate with
Q42: Which of the following equation best represents
Q44: What is the net ionic equation for
Q45: _-oxides produce acids when reacted with water.
Q46: Which of the following compounds is soluble
Q47: Identify a true statement about oxidation numbers.<br>A)
Q48: A solution is a homogeneous mixture composed