Multiple Choice
Look at the bond energies O-H, N-H, and P-H in the table below. O-H is the hardest bond to break because it has the 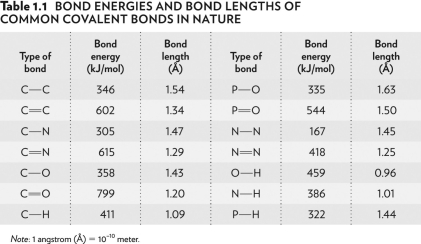
A) greatest difference in relative affinities of the two atoms for electrons.
B) smallest difference in relative affinities of the two atoms for electrons.
C) smallest difference in atomic size.
D) largest difference in atomic size.
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Hydrogen bonds form between hydrogen and<br>A) oxygen.<br>B)
Q5: The "central dogma of molecular biology" can
Q24: In DNA the phosphate groups are on
Q35: What is the cause of the overall
Q46: Which of the following is an example
Q48: The study of biochemistry attempts to explain<br>A)
Q61: Which six elements make up 97% of
Q81: Which two functional groups are involved in
Q83: A genome is a set of<br>A) proteins.<br>B)
Q95: Mutations to proteins typically occur starting<br>A) at