Multiple Choice
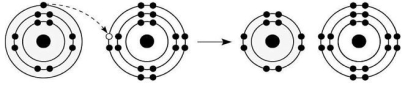 Figure 2.6
Figure 2.6
-What results from the chemical reaction illustrated in Figure 2.6?
A) formation of a covalent bond
B) formation of an ionic bond
C) formation of a hydrogen bond
D) formation of an anion with a net charge of +1 and a cation with a net charge of -1
Correct Answer:

Verified
Correct Answer:
Verified
Q120: What is the pH of a solution
Q121: Molybdenum has an atomic number of 42.Several
Q122: An equal volume (5 mL)of milk of
Q123: Consider two solutions: solution X has a
Q124: Unequal sharing of electrons between atoms will
Q126: The atomic number of sulfur is 16,which
Q127: What is the hydroxyl ion (OH<sup>-</sup>)concentration of
Q128: In ammonium chloride salt (NH<sub>4</sub>Cl),the anion is
Q129: Buffers are substances that help resist shifts
Q130: The molar mass of glucose (C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>)is 180