Multiple Choice
Which of the atoms shown would be most likely to form a cation with a charge of +1?
A) 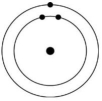
B) 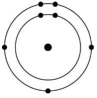
C) 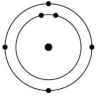
D) 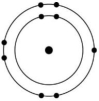
E) 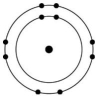
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q57: The molar mass of glucose is 180
Q59: The nutritional information on a cereal box
Q60: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7910/.jpg" alt=" Figure 2.4 -How
Q61: Why does ice float in liquid water?<br>A)The
Q62: One liter of a solution of pH
Q63: Increased atmospheric CO<sub>2</sub> concentrations will have what
Q65: If acid rain has lowered the pH
Q66: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7910/.jpg" alt=" Figure 2.8 -How
Q67: How many glucose molecules are contained in
Q68: A beaker contains 100 mL of NaOH