Multiple Choice
A small birthday candle is weighed.It is then lighted and placed beneath a metal can containing 100 mL of water.Careful records are kept as the temperature of the water rises.Data from this experiment are shown on the graph in Figure 2.9.What amount of heat energy is released in the burning of candle wax? (Note that 1 liter of pure water has a mass of 1 kg. ) 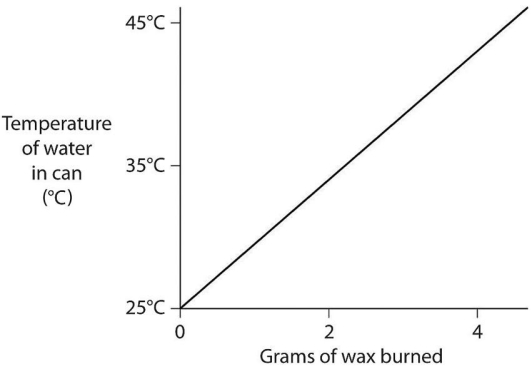 Figure 2.9
Figure 2.9
A) 0.5 kilocalorie per gram of wax burned
B) 5 kilocalories per gram of wax burned
C) 10 kilocalories per gram of wax burned
D) 20 kilocalories per gram of wax burned
E) 50 kilocalories per gram of wax burned
Correct Answer:

Verified
Correct Answer:
Verified
Q42: Which of the following takes place as
Q96: Which bond or interaction would be most
Q97: Which of the atoms shown would be
Q99: If the cytoplasm of a cell is
Q100: Given only a mass number,one can deduce
Q102: How many molecules of glycerol (C<sub>3</sub>H<sub>8</sub>O<sub>3;</sub>molecular mass
Q103: If the pH of a solution is
Q104: How does the formation of covalent bonds
Q105: What is the hydrogen ion (H<sup>+</sup>)concentration of
Q106: An atom has 6 electrons in its