Multiple Choice
Identify the hybridization of carbon atoms numbered 1-6 in the structure below: 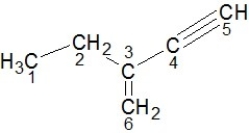
A) Carbons 1 and 2 are sp3, carbons 3 and 6 are sp2, carbons 4 and 5 are sp hybridized.
B) Carbons 1 and 3 are sp3, carbons 2 and 5 are sp2, carbons 4 and 6 are sp hybridized.
C) Carbons 2 and 3 are sp3, carbons 4 and 5 are sp2, carbons 1 and 6 are sp hybridized.
D) Carbons 4 and 5 are sp3, carbons 3 and 6 are sp2, carbons 1 and 2 are sp hybridized.
E) Carbons 3 and 6 are sp3, carbons 4 and 5 are sp2, carbons 1 and 2 are sp hybridized.
Correct Answer:

Verified
Correct Answer:
Verified
Q46: Which of the bold and underlined carbon
Q47: How many of the carbons in the
Q49: How many of the carbons in the
Q50: Which of the following structures is R-but-3-en-2-ol?<br>A)
Q52: Which of the following statements is TRUE?<br>A)
Q53: Which of the following compounds is ethanol?<br>A)
Q54: What are the possible combinations of multiple
Q55: Which compound is a saturated hydrocarbon?<br>A) 1-butyne<br>B)
Q56: Which of the following structures shows a
Q71: Which one of the following molecules is