Multiple Choice
Identify hybridization of carbon atoms numbered 1-6 in the structure below: 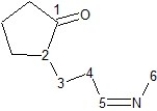
A) Carbons 3, 4, and 6 are sp3, carbons 1, 2 and 5 are sp hybridized.
B) Carbons 2, 3, and 4 are sp3, carbons 1, 5, and 6 are sp2 hybridized.
C) Carbons 2, 3, 4, and 6 are sp3, carbons 1 and 5 are sp2 hybridized.
D) Carbons 2, 3, 4, and 6 are sp3, carbons 1 and 5 are sp hybridized.
E) Carbons 3, 4, and 6 are sp3, carbons 1, 2, and 5 are sp2 hybridized.
Correct Answer:

Verified
Correct Answer:
Verified
Q62: Which of the following lists the substituents
Q63: Match the following.<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7901/.jpg" alt="Match the following.
Q64: Which of the following compounds exhibit cis-trans
Q65: Which of the following compounds is an
Q66: Which of the following condensed general formulas
Q68: Determine the absolute configuration of the molecule
Q69: The infrared spectrum of a compound with
Q70: Which of the following pairs of organic
Q71: Explain the concept of bond polarization and
Q72: Find the correct Newman projection formula showing