Multiple Choice
Write a nuclear equation for the alpha decay of 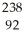 U.
U.
A) 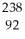 U →
U → 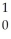 n +
n + 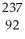 U
U
B) 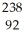 U →
U → 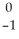 e +
e + 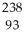 Np
Np
C) 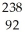 U →
U → 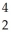 He +
He + 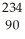 Th
Th
D) 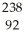 U →
U → 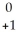 e +
e + 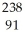 Pa
Pa
E) 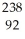 U →
U → 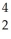 He +
He + 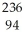 Pu
Pu
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: Identify the nuclide that has the shortest
Q9: The radioactive decay of _ is the
Q10: Which radiation has the highest penetrating power?<br>A)
Q11: Determine how many neutrons are produced during
Q14: Above what atomic number are there no
Q15: Which of the following statements is TRUE?<br>A)
Q16: Match the following.<br>-positron<br>A)<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7901/.jpg" alt="Match the following.
Q17: Describe what changes occur in the atomic
Q18: Identify the instrument used to detect radiation.<br>A)
Q39: Which of the following nuclides are most